Nov. 12, 2024
VCU surgeon’s innovation is finding a quick stick in top operating rooms across the country
Share this story
This may be the surgical equivalent of a musical act being discovered – and making it big.
At the American Society for Reconstructive Microsurgery annual meeting in January this year, a Mayo Clinic breast surgeon stumbled upon a live demonstration of Nerve Tape. The product – a tiny biologic wrap that repairs severed peripheral nerves – was developed by a Virginia Commonwealth University hand surgeon.
“I was very excited about it,” said Alanna Rebecca, M.D., a plastic surgeon and physician leader in Mayo’s breast clinic who saw the potential for Nerve Tape in her Arizona operating rooms. “It’s one of those straightforward ideas that works really well. It just makes logical sense.”
What began as a conference eye-opener gained momentum with support from teams at Mayo. This summer, Rebecca became of the first surgeons to implant Nerve Tape – and buoyed by an impressive 2024 so far, it could become one of VCU’s most successful licensing deals ever through its innovation pipeline.
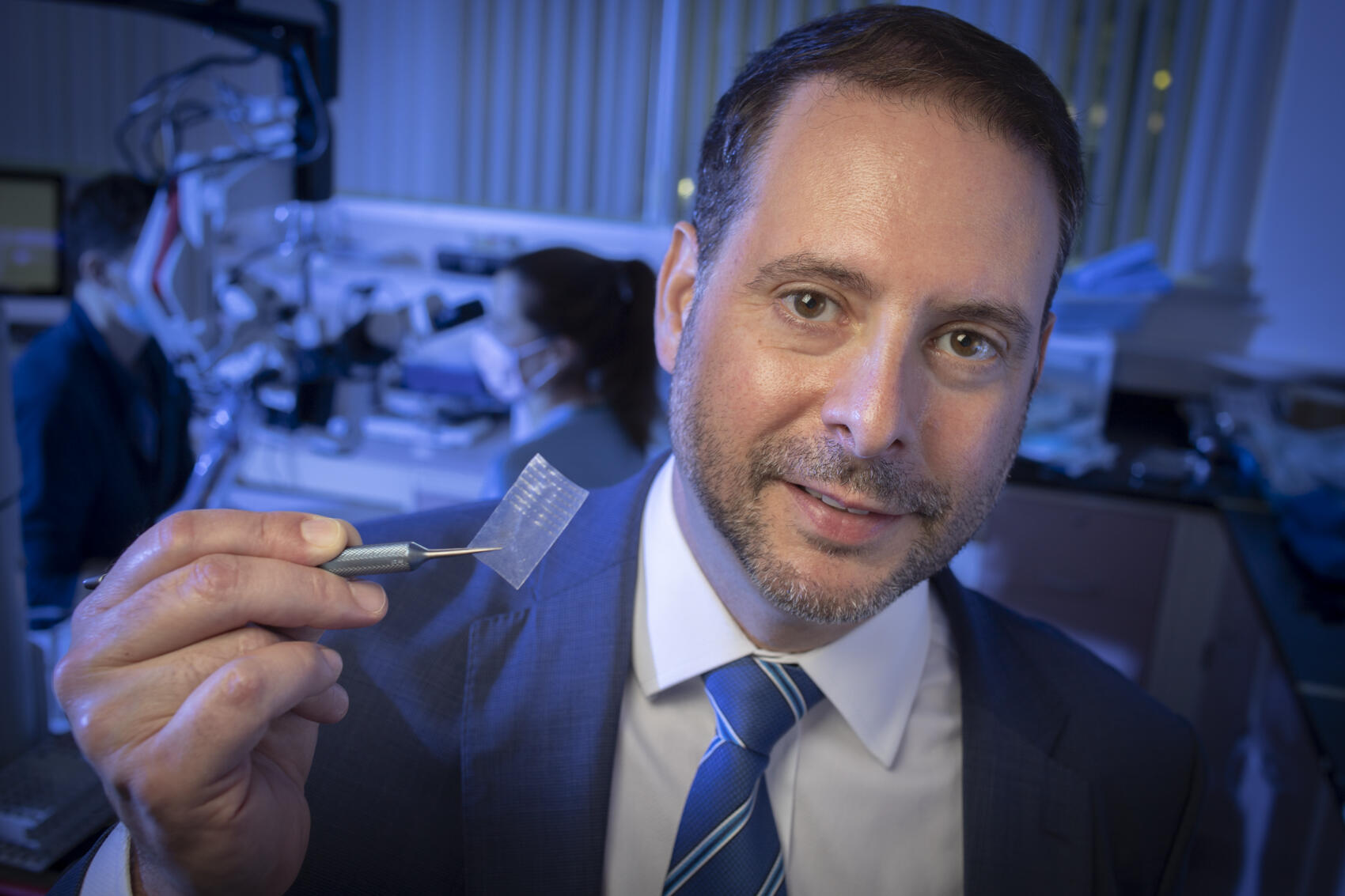
The development of Nerve Tape began with Jonathan Isaacs, M.D., a professor in the VCU School of Medicine’s Department of Orthopaedic Surgery and chair of VCU Health’s Division of Hand Surgery. He wanted to improve nerve repair, whose traditional suturing techniques required precise alignment and significant time.
Isaacs envisioned a simpler solution: a biologic wrap to streamline and enhance the nerve repair process. Like a piece of high-tech tape with tiny, flexible embedded hooks, Nerve Tape loops around and self-seals a nerve’s outer connective tissues, improving nerve alignment and promoting regeneration.
“The initial concept was, could we use Nerve Tape as a delivery device? And then, if we’re going to bother to do this, why don’t we just make it sticky, and it will hold the nerve ends together,” Isaacs said. “I recognized very early that I needed a mechanical hold to the nerve, and that’s really where the concept that turned into Nerve Tape came from.”
A major impact in medical care
Turning the idea into a workable product required significant collaboration and technical development. Isaacs partnered with BioCircuit Technologies (previously known as Axion), and VCU TechTransfer and Ventures supported his team to secure patents and negotiate a licensing agreement with BioCircuit.
“There is palpable enthusiasm among surgeons both in Virginia and around the country,” Isaacs said. “There is fair reason to be very optimistic that this is going to make a big difference in the future of nerve repair.”
At Mayo Clinic, breast specialist Rebecca quickly saw Nerve Tape’s potential to be a game-changer for mastectomy patients.
“There’s been quite a bit of research showing that not having sensation is a detriment to quality of life for women who’ve had a mastectomy,” she said. “The benefit of actually bringing that sensation back is amazing.”
Rebecca said she has always prioritized nerve reconnection, or neurotization, but many surgeons don’t routinely reconnect nerves because of the complexity and time required. Anything to make operating less tedious and technical would be a win for doctors and patients, and after seeing the Nerve Tape demonstration this year, she not only established ongoing contact with BioCircuit – she started preparing her Arizona team.
Rebecca closely tracked Nerve Tape’s data and its approaching clearance by the Food and Drug Administration. She coordinated with her hospital’s procurement and supply departments to ensure they were ready when Nerve Tape for surgical use was available.
“We had our operating room and surgical supply team prepped, waiting for things to be approved, so we had already done all the paperwork,” she said.
BioCircuit announced this summer that Rebecca become one of the first surgeons to implant Nerve Tape.
“It’s been an amazing product. It cuts about 15 to 20 minutes per side for me, so it’s about a half hour to 45 minutes of surgical time saved,” she said. “Less anesthesia means patients are getting out sooner, and it’s cost-effective.”
Attention at the national level
Nerve Tape’s innovative approach to sutureless nerve repair has caught the attention of the medical community as well as federal institutions. The product was featured in the 2024 Congressional Budget Justification for the National Institute of Neurological Disorders and Stroke, highlighting it as an example of impactful research funded by the National Institutes of Health.
And it was only in mid-April that BioCircuit made its first commercial sale of Nerve Tape, a milestone in the product’s journey from development to reaching operating rooms.
BioCircuit CEO Michelle Jarrard said the company crafted a phased commercial launch designed to prioritize patient safety and surgeon education. For initial adopters, the company focused on leading academic medical centers across the U.S. It’s their surgeons who quickly have been advocating for Nerve Tape in their health systems and are accelerating its use.
“Every time I hear from anyone who’s tried it, it’s positive,” Rebecca said of surgical colleagues.
And as Jarrard notes, “we are fortunate to have surgeon adoption – which is no small task.”
She said that as of September, BioCircuit had exceeded expectations with its rollout approach, and “each month has been bigger than the last so far.”
Another good early sign is retention: Every customer that has used Nerve Tape has reordered it. “We’ve sold hundreds and hundreds and hundreds of devices,” said Jarrard, with specifics remaining confidential.

Exploring a promising future
Amid Nerve Tape’s acceptance and growing spotlight, Jarrard said the product has entered a new chapter.
“As of the end of August, BioCircuit declared victory on its rollout phase of Nerve Tape,” she said. “It is now available to all interested surgeons and institutions in the U.S.”
BioCircuit already is looking to additional applications for Nerve Tape, and for a technology that started with a VCU surgeon’s vision, the growing enthusiasm from colleagues across the U.S. is shaping a promising future. With its efficacy established in upper extremity and breast reconstruction surgeries, surgeons are exploring its prospects for lower extremity repairs, sarcoma surgeries and more.
In reflecting on his journey, Isaacs emphasized the mix of perseverance and potential.
“If you believe in your ideas, stick with it,” he said. “I would argue one of the reasons why we’ve been so successful is because we had a problem that we came up with a solution for – not the other way around. And looking ahead, this technology that we, as a team, developed has a lot of possibilities.”
Indeed, “team” is a key element, with TechTransfer and Ventures helping innovators raise VCU’s standing as one of the nation’s leading public research universities.
“Our goal is to support the journey from idea to implementation, ensuring that groundbreaking innovations like Nerve Tape reach their full potential,” said Ivelina Metcheva, Ph.D., VCU’s assistant vice president for innovation and head of TechTransfer and Ventures.
“By working closely with innovators like Dr. Isaacs, we are able to transform novel concepts into market-ready solutions that meet real clinical needs and have a profound impact on patient care,” she added. “Nerve Tape serves as a bright example of the benefits of a strong universitywide ecosystem of innovation, licensing and development. We’re excited about what the future holds for this innovative device and VCU.”
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.







