Nov. 9, 2020
Finding treatments for muscular dystrophy starts with understanding the condition better
Share this story
Muscular dystrophy, a condition that affects the body’s muscles, comes with progressive muscle weakness and prolonged periods of muscle tightness. An estimated 975,000 to 3 million people worldwide have been diagnosed with the most common type of muscular dystrophy, myotonic dystrophy type 1.
A study led by a VCU Health physician and researcher aims to increase scientists’ and health professionals’ understanding of myotonic dystrophy type 1 and support the development of new treatment options for the condition, which has no cure.

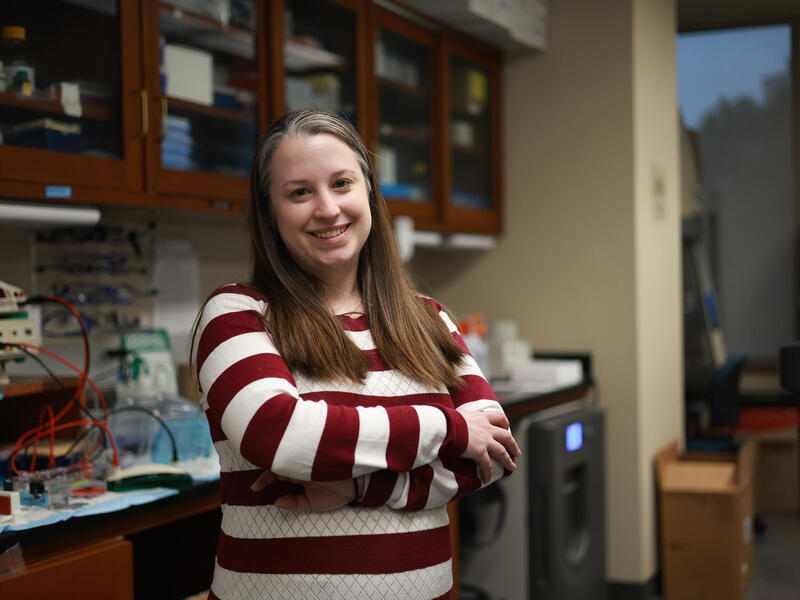
Nicholas Johnson, M.D., an associate professor and vice chair of research in the Department of Neurology at VCU School of Medicine, serves as co-primary investigator for the international study, “Establishing Biomarkers and Clinical Endpoints in Myotonic Dystrophy Type 1” (END-DM1), along with co-primary investigator Charles Thornton, M.D., at the University of Rochester Medical Center.
Johnson, a neurologist at VCU Health, has led the development of multiple clinical trials for myotonic dystrophy and other muscular dystrophies as part of a team at VCU Health working to advance the treatment of genetic muscle disorders.
By 2024, the END-DM1 project is expected to enroll about 700 patients with myotonic dystrophy type 1 across 16 study sites in the U.S. and Europe. The project has received more than $8.5 million in funding from the U.S. Food and Drug Administration, the Myotonic Dystrophy Foundation, the Wyck Foundation, the Muscular Dystrophy Association, Dyne Therapeutics and the National Institutes of Health’s National Center for Advancing Translational Sciences.
Johnson described this new study and how it has the potential to make a difference for people with myotonic dystrophy type 1.
What is myotonic dystrophy type 1, and how does it typically affect patients?
Myotonic dystrophy type 1 affects the skeletal muscle causing weakness but also affects many other organ systems and may cause the development of cataracts, cardiac arrhythmias, breathing problems, diabetes, thyroid problems, brain dysfunction and excessive daytime sleepiness. It is progressive, leads to early death and is not currently treatable.
Tell me about this clinical trial. What is the goal, and what is VCU doing to lead this END-DM1 clinical trial?
The END-DM1 study is a natural history study designed to: 1) understand the progression of myotonic dystrophy; 2) refine our future therapeutic trial protocols to better capture those patients with a more rapid progression; and 3) optimize the biomarkers for myotonic dystrophy for clinical trials. VCU serves as the coordinating center for this study, which includes overall study conduct, site management and supporting enrollment at the sites. University of Rochester is the co-coordinating site and the data center.
How will this project help patients?
Prior drug studies have been limited by a lack of understanding of the disease, preventing the ability to truly test whether these therapies are effective. This study will allow for the optimization of clinical trials in myotonic dystrophy and a better understanding of the disorder overall. With approaching therapeutic agents, this is of urgent importance so that novel precision-based therapies could be tested appropriately.
How can people enroll in this study?
We need participants to continue to enroll. People ages 18 to 70 with myotonic dystrophy type 1 may qualify for this study. More information and study team contact information are available at studyfinder.cctr.vcu.edu by searching “Establishing Biomarkers and Clinical Endpoints in Myotonic Dystrophy Type 1,” or at clinicaltrials.gov.
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.