March 18, 2025
VCU researchers are developing a long-acting medication for opioid addiction
Share this story
A
s the United States grapples with an unrelenting opioid crisis, researchers at Virginia Commonwealth University have reformulated an opioid use disorder medication in a way that could extend its therapeutic effect – and offer a longer-lasting pharmaceutical therapy for treating opioid addiction.
In 2023, more than 150 people died every day on average from opioid overdose in the U.S., according to the Centers for Disease Control and Prevention. A number of medications help curb opioid addiction, but several barriers can interfere with a patient’s path to recovery, such as strict regulations, adverse side effects and limited access to treatment clinics.
In hope of providing more treatment options for opioid use disorder, the VCU research team reworked nor-levo-alpha-acetylmethadol, a metabolite of a previous FDA-approved opiate dependence medication, into a new formulation that could be used to help patients with opioid addiction. While existing medications require daily doses, this new formulation is designed to be a long-acting alternative, requiring doses only once a month or potentially at even longer intervals.
Their latest study, published in the Journal of Controlled Release, showed that the reformulated medication significantly reduced opioid use and withdrawal symptoms in rodent models. The researchers say these findings have promising implications for ultimately expanding the range of medicinal therapies available for treating opioid addiction.
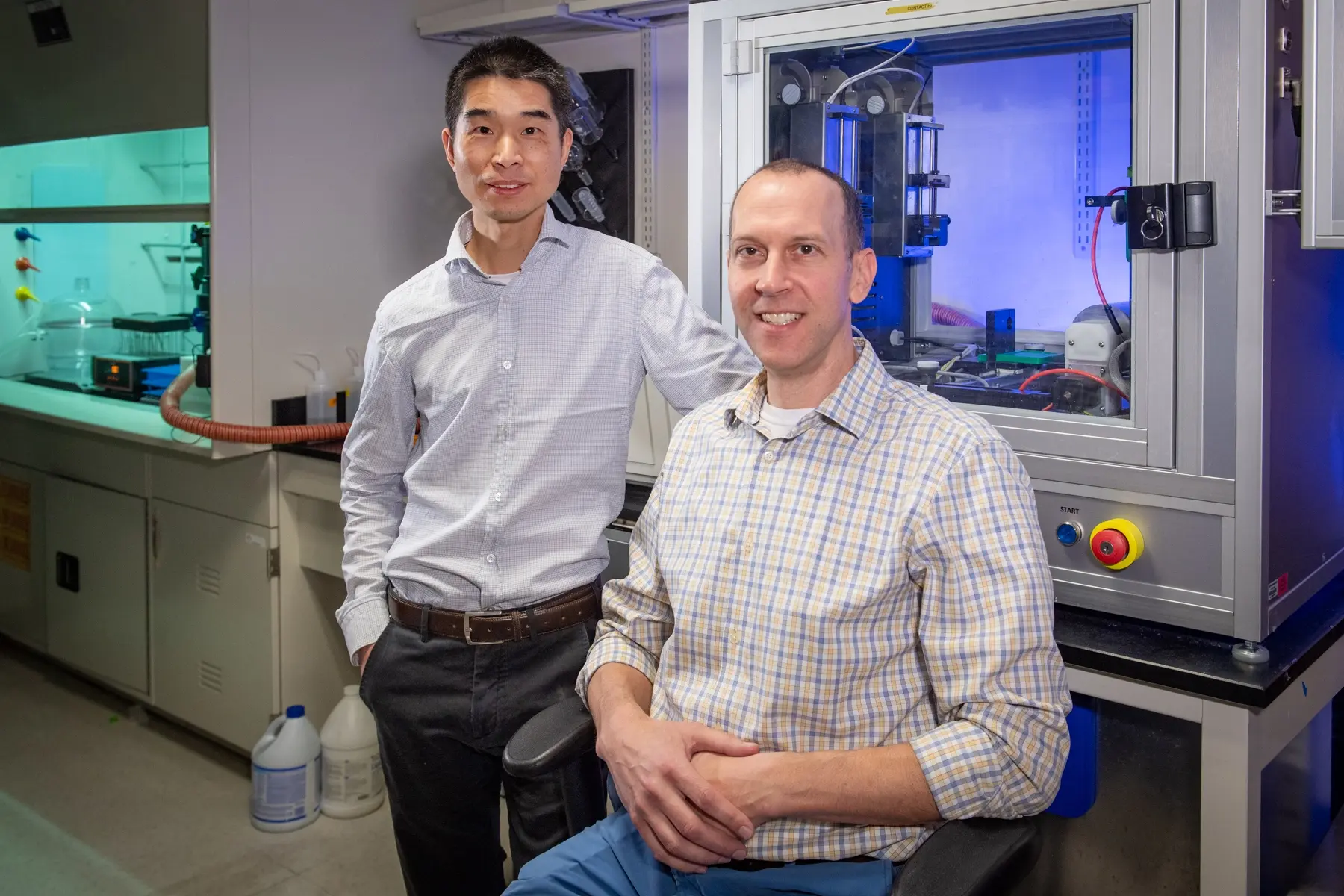
“There is an urgent need to develop more therapeutic strategies for enhancing the effectiveness of our interventions and the overall well-being of patients with opioid use disorder. Our goal is to give physicians another tool in their toolbox to help patients overcome addiction,” said Qingguo Xu, Ph.D., an associate professor in the VCU School of Pharmacy’s Department of Pharmaceutics. He co-led the new study with Matthew Banks, Ph.D., a professor in the VCU School of Medicine’s Department of Pharmacology and Toxicology.
There are currently three FDA-approved medications used to treat opioid use disorder: methadone, buprenorphine and naltrexone. Despite their effectiveness in helping patients curb their addiction, barriers to access and other challenges still persist.
“Part of the issue is that each of these medications comes with their own set of regulatory hurdles. In particular, methadone is very effective at decreasing illicit drug-taking behavior, but there are a lot of regulatory restrictions and stigma around its use,” Banks said. “Another challenge is that some patients are unresponsive to the current medications or experience undesirable side effects.”
“Some patients also have a hard time managing the dose requirements of the current medications,” Xu added. “Methadone treatment requires daily visits to an approved methadone clinic, which can be a huge burden for patients, especially if they live in rural areas with limited access to clinics. Since the pandemic, government officials have been working to loosen those restrictions.”
To further address these issues, Xu and Banks have turned to levo-alpha-acetylmethadol, also known as LAAM. The medication was approved by the Food and Drug Administration in 1993 for treating opioid dependence, and previous research had shown LAAM to be more effective than methadone in suppressing opioid use. However, LAAM was taken off the market in 2003 due to concerns about cardiac effects and declining sales after buprenorphine’s introduction as a prescription treatment.
The VCU-led research team created a reformulated version of this medication, which they call nor-LAAM, and initial studies suggest it could be a safer, more potent alternative to LAAM. Their goal is to give pharmaceutical companies a reason to bring back the medication as an additional option for reducing opioid cravings and preventing relapse.
For this new formulation, the researchers developed a novel drug-loading system that packs a high dose of nor-LAAM into biodegradable microparticles, which in turn releases a steady level of the medication over a long period of time. While current medications for opioid use disorder often require daily doses, nor-LAAM is designed to be taken once a month, or potentially even less frequently.
“This is an important benefit since reducing the frequency of doses can make it easier for patients to comply with their treatment plans,” Banks said.
He and Xu have since been leading preclinical studies to better understand nor-LAAM’s potential for treating opioid use disorder. In their latest study, the researchers examined the behavior of fentanyl-dependent rodents when treated with either nor-LAAM or a placebo. They specifically looked into how the medication impacted the subjects’ preferences when given the option to either self-administer fentanyl or receive food.
Their study revealed that subjects treated with nor-LAAM significantly reduced their preference for fentanyl over food over the course of four weeks. Additionally, subjects treated with nor-LAAM exhibited fewer signs of opioid withdrawal over time.
While this project is still in the preliminary stages and it may be a few more years until this medication is ready for human clinical trials, the researchers say these findings give promising insights into nor-LAAM’s potential as a long-acting strategy for treating opioid addiction. Looking ahead, Xu and Banks will continue to develop nor-LAAM formulations and assess its effectiveness for therapeutic use.
“We hope that expanding the medicinal options for opioid use disorder helps with increasing patient retention to their treatments, gives them a better chance to overcome addiction and ultimately gives them back to their lives,” Xu said.
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.