Feb. 14, 2007
A new approach to anti-cancer drug design
Share this story
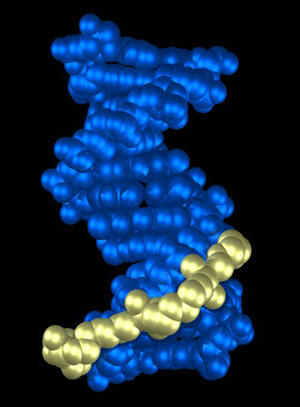
A Virginia Commonwealth University research team, led by Nicholas P. Farrell, Ph.D., professor and chair in the Department of Chemistry, has uncovered a distinct third mode of DNA binding that may help researchers design new drugs to fight cancer.
Farrell’s group made the finding while working to improve the action of cisplatin, one of the most effective anti-cancer drugs in the clinic. Discovery of the new mode of DNA binding is the first since two scientists examining drug interactions with DNA made separate discoveries in the 1960s and 1970s that set the standard for drug design efforts.
Forty years ago, Leonard Lerman, Ph.D., a molecular biologist, found that through a method known as intercalation, certain molecules could bind to DNA. This was followed a decade later by the discovery of a second mode of DNA-binding, called minor groove association, proposed by scientist, Robert Wells, Ph.D.
Their work shaped the understanding and development of important drug and therapeutic strategies to fight a variety of diseases – including cancer.
This new, third mode of DNA binding may help researchers design a new generation of platinum-based, anti-cancer drugs that are less toxic, reduce resistance and side effects, and expand the range of tumors that can be treated by platinum. The team included researchers from the VCU Department of Chemistry and the VCU Massey Cancer Center.
According to Farrell, this new mode of DNA binding exclusively utilizes interactions of the functional groups of the molecule with the DNA backbone. Farrell said that the mode of binding is made solely through interactions with the DNA backbone. The platinum-based molecules “track” the backbone of DNA through a repetitive motif.
“Our findings elicit a new way of thinking when it comes to designing DNA-binding anti-cancer drugs. We have shown a third mode of binding discrete from those previously proposed. This is the first genuinely new mode of DNA binding to be described using a technique known as X-ray crystallography,” said Farrell. The findings were published early online in the Dec. 2, 2006, issue of the Journal of the American Chemical Society.
Cisplatin, which is a mononuclear platinum-containing compound, works by attaching itself to DNA, and then slows or stops the growth of cancer cells in the body. Damage to DNA causes tumor cells to switch-on a cell suicide mechanism, hence altering the ability for the cells to divide rapidly. DNA-damaging agents, such as cisplatin, are among the most effective classes of compounds in clinical use for the treatment of cancer. Of these, platinum drugs are the largest class in the clinic and the most important in terms of treatment. They are first-line treatment for metastatic testicular and ovarian cancers, as well as metastatic colon cancer.
However, according to Farrell, these current agents have limited activity against many common human cancers, and they are susceptible to acquired drug resistance. He added that resistance to cisplatin has become a clinically relevant issue - especially for patients battling ovarian cancer because they develop resistance to cisplatin at a rapid rate.
In 2005, Farrell and his team created a new platinum-based, anti-cancer agent able to overcome acquired drug resistance by first modifying the way it is absorbed into cancer cells and then attacking the DNA of those cancer cells.
(http://pubs.acs.org/cgi-bin/article.cgi/inocaj/2005/44/i26/pdf/ic051390z.pdf)
“The novel compound identified in our previous work was designed to overcome resistance by emphasizing new modes of DNA binding. Now we have a three-dimensional picture of exactly how the DNA is attacked,” Farrell said. “This now allows us to further make rational design choices to enhance activity.”
The work was supported by the National Institutes of Health and by the Japan Society for the Promotion of Science.
Farrell collaborated with VCU researcher Seiji Komeda, Ph.D.; Loren Dean Williams, Ph.D., Tinoush Moulaei, Ph.D., and Kristen Kruger Woods, Ph.D., all with the School of Chemistry and Biochemistry at Georgia Institute of Technology; and Masahiko Chikuma, Ph.D., with the Osaka University of Pharmaceuticals Sciences.
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.







