Nov. 22, 2006
Extending the Periodic Table of Elements
Introducing multiple valence superatoms
Share this story
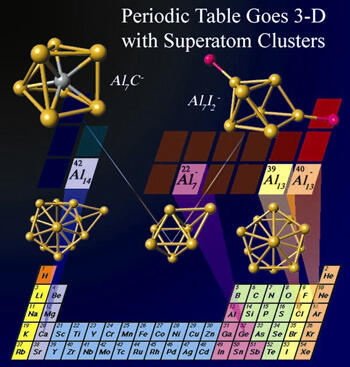
A team of Virginia Commonwealth University and Penn State researchers that last year discovered a new form of chemistry is another step closer to extending the periodic table to a third dimension.
Through theoretical work, Shiv N. Khanna, Ph.D., professor in physics at VCU, and his team have identified a new class of multiple valence ‘superatoms’ with unique chemical and electronic behaviors. The theoretical effort is complemented by the experimental work by researchers led by A.W. Castleman Jr., Ph.D., a professor at Penn State University.
In previous research findings, Khanna, together with Penn State colleagues, had demonstrated that aluminum clusters can act as halogen or alkaline earth elements, thus allowing for the creation of new families of nanoscale materials with extraordinary attributes.
According to Khanna, the production and stabilization of such species is a stirring development as it opens a new branch of chemistry and material science, showing that these “superatoms” can be used as building blocks to form new nanoscale materials that could lead to new applications in medicine, catalysis, sensors and other fields.
The term valence refers to the number of chemical bonds an atom can form or the number of electrons an atom donates or accepts when reacting to form compounds. An atom is in a stable configuration when its outermost shell is full. Consequently, when an atom combines with other atoms, it tends to lose or gain valence electrons to acquire a stable configuration.
Khanna said that one of the most important features of elements in the periodic table is that selected atoms exhibit multiple valences. For example, a carbon atom has a valence of two and four. Carbon forms a stable molecule with a single oxygen atom, resulting in carbon monoxide (CO), and with two oxygen atoms, resulting in carbon dioxide (CO2).
“We wanted to find out if superatoms share this commonality with the elements of the periodic table. We have shown that a particular class of superatoms can exhibit multiple valences and that it can form stable compounds when combined with elements that require two or four electrons to fill their shells,” said Khanna.
Khanna and his colleagues looked at a class of superatoms, namely Al7-, and combined it with atoms in the second, third and fourth rows of the periodic table. They found that the most stable compounds formed with atoms that require two and four electrons to fill their electronic shells. They also proved this multiple valence theory by combining Al7- with two and four iodine atoms. The work was recently published in the Proceedings of the National Academy of Sciences Online Early Edition the week of Nov. 20.
Khanna collaborated with J. Ulises Reveles, Ph.D., a research associate in Khanna’s group in the Department of Physics at VCU; and Castleman and P.J. Roach, a research associate in the Departments of Chemistry and Physics at Penn State University.
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.