March 9, 2021
The Johnson & Johnson vaccine is here. Health experts say you should get it if offered.
Share this story
Editor’s note (May 3, 2021): In keeping with the recent decision by the CDC and FDA, VCU Health has lifted the temporary pause it placed on administering the Johnson & Johnson vaccine. We are now administering all three vaccines — Pfizer, Moderna and Johnson & Johnson. This content is accurate as of the update date. For the most up-to-date information on this topic, please visit the VCU Health COVID-19 vaccine page.
Millions of people have now received COVID-19 vaccines in the United States. On Feb. 27, the Food and Drug Administration granted emergency use authorization to Johnson & Johnson’s vaccine, making it the third now available in the U.S., joining vaccines from Moderna and Pfizer. On March 2, President Biden said the U.S. will have enough vaccine supply to inoculate all adults by May. And on March 8, the Virginia Department of Health expanded who is eligible to receive the COVID-19 vaccine.
As more doses become available, infectious disease experts stress the importance of people getting vaccinated as soon as they can — no matter which vaccine they are offered.
“Get what you can get when something becomes available to you,” said Michael Stevens, M.D., associate chair of the Division of Infectious Diseases at the Virginia Commonwealth University School of Medicine. “If you have access to get any vaccine, you should get it."
Virginia is currently distributing vaccines for those in phases 1a and prioritized 1b categories. VCU faculty and staff who are not eligible as part of phase 1a or 1b will be eligible as part of phase 1c. VCU students, unless otherwise eligible as part of phases 1a, 1b or 1c, will be eligible when the vaccine becomes widely available for the general population.
As with the Moderna and Pfizer vaccines, the Johnson & Johnson vaccine is effective against COVID-19. In clinical trials of nearly 44,000 people the vaccine was found to be “85% effective in preventing severe/critical COVID-19 occurring at least 28 days after vaccination,” according to the FDA.
While the Moderna and Pfizer vaccines that were authorized earlier this winter have higher overall rates of effectiveness against the virus, the Johnson & Johnson vaccine could be the game changer that marks a turning point in the pandemic. The Johnson & Johnson vaccine is still very effective in preventing severe disease, and that “is really what you want from a vaccine,” Stevens said.
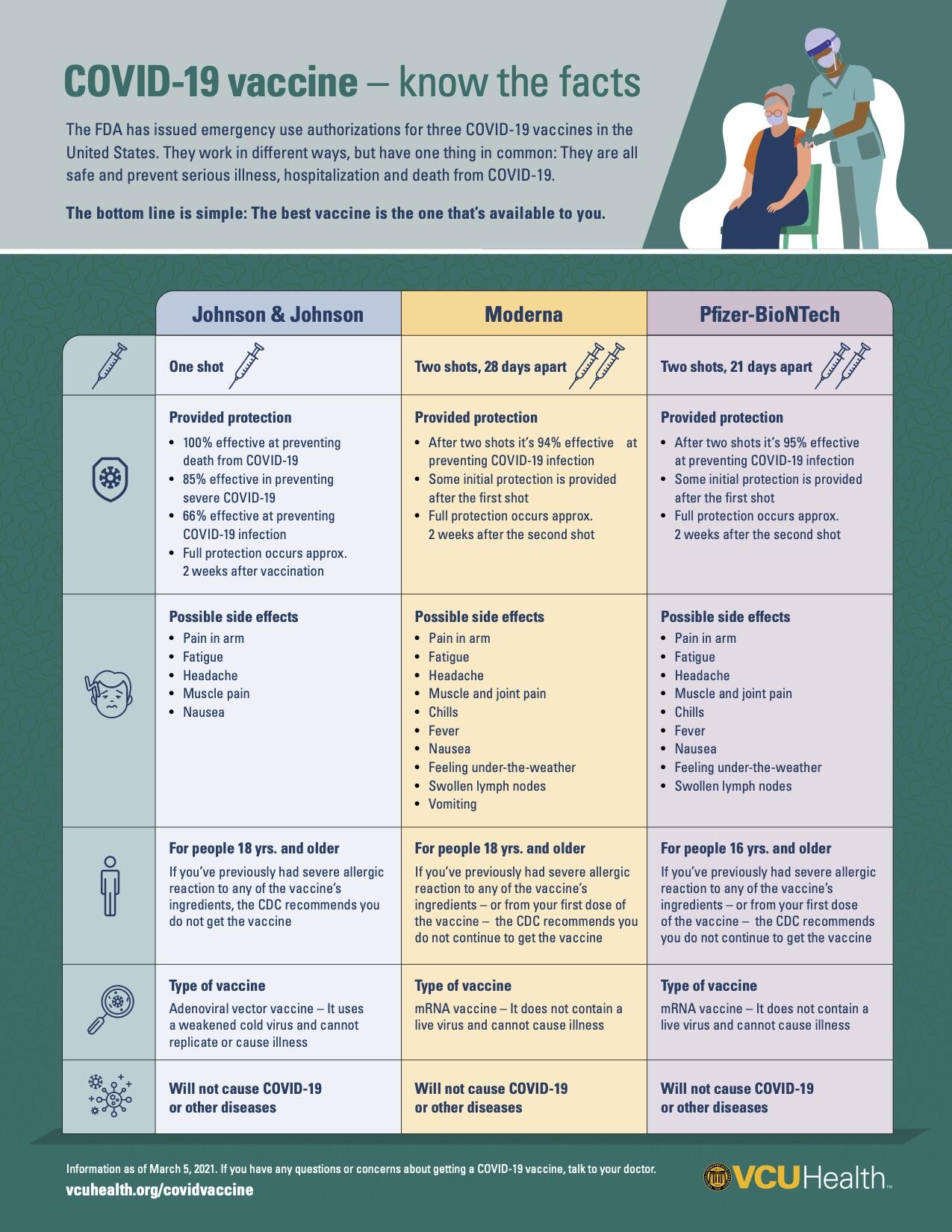
The Johnson & Johnson vaccine, Stevens said, “is a more traditional vaccine approach,” in which the vaccine uses a harmless virus that can’t replicate to prompt the body to have an immune response. The Pfizer and Moderna vaccines, meanwhile, use mRNA technology. While safe and effective, those vaccines require extra hurdles regarding storage and distribution compared to the Johnson & Johnson vaccine.
“The huge advantage to the Johnson & Johnson vaccine is that it is a single shot. The mRNA vaccine requires two shots,” Stevens said. “As well, [the Johnson & Johnson vaccine] can be kept at essentially refrigerator temperatures for months, and it’s stable. The mRNA vaccines require more sophisticated cold storage and they are not as stable.
“When you start talking about a vaccine that is more stable and only involves a single shot, this is great news,” he said.
All three vaccines have gone through a rigorous process at the FDA to receive emergency use authorization, he said.
“If it has emergency use authorization from the FDA, it has been held to a very high safety standard,” Stevens said.
His overall message: Get vaccinated as soon as you can.
“The more vaccines in arms the better,” Stevens said. “The more people who are vaccinated, the faster we will get out of this mess.”
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.