March 24, 2020
VCU researchers begin clinical trials on investigational drug therapy for COVID-19
Share this story
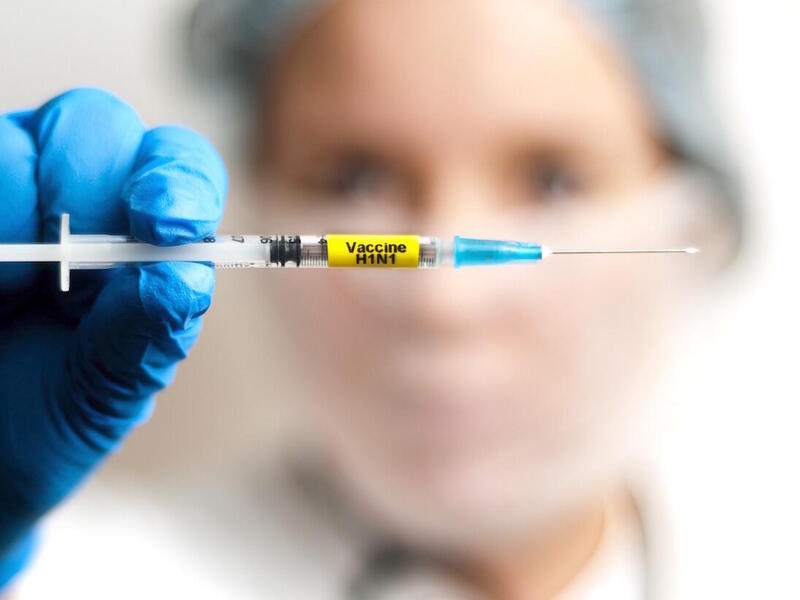
Virginia Commonwealth University researchers began two clinical trials this week on a potential, experimental treatment for COVID-19, the novel coronavirus rapidly spreading across the globe.
Arun Sanyal, M.D., a liver specialist and gastroenterologist at VCU Health, is leading clinical trials of an investigational drug for patients with moderate and severe symptoms of COVID-19 and the virus responsible for the disease, SARS-Cov-2.
The investigational antiviral drug remdesivir was developed by Gilead Sciences Inc. and used experimentally to treat Ebola. Earlier this year, its use on a man hospitalized with coronavirus in Washington state piqued the interest of researchers globally. Remdesivir is an investigational agent — it is not approved anywhere globally and has not been demonstrated to be safe or effective for any use.
“We feel it is extremely important that drugs to be used for this potentially life-threatening disease be tested rigorously so we have good evidence of their efficacy as well as their safety,” Sanyal said. “We are delighted to work with Gilead on this trial, and we look forward to generating the data that hopefully will help lots of people who have this condition.”
VCU is one of the handful of institutions in the United States to make these clinical trials available to patients who meet the criteria for this investigational drug.
“The selection of VCU as a site for this global trial reflects our ability to bring multidisciplinary care to clinical trials and in having the capacity, the breadth and the depth of expertise needed to manage these patients,” said Sanyal, a professor of Internal Medicine in the VCU School of Medicine.
Remdesivir has previously shown antiviral activity against other coronaviruses like Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) in vitro and in animal models. But clinical trials were never completed due to the lack of available study participants, and the investigational drug is not currently publicly available.
Remdesivir acts by mimicking the appearance of part of the virus and infiltrating the enzyme that viruses can use to replicate themselves. It is designed to slow the infection of healthy cells in a patient’s body.
The trials are a chance not only to provide access to remdesivir, Sanyal said, but also to generate data on the investigational drug’s safety and effectiveness.
VCU’s trials will enroll participants with documented COVID-19 infection who have fever and symptoms warranting hospitalization. Doctors at VCU Health will administer the investigational drug intravenously in five- or 10-day regimens and follow up 28 days later. There are two levels of acuteness being tested: moderate and severe, the latter defined as “someone whose symptoms require them to be on supplemental oxygen,” Sanyal said.
We feel it is extremely important that drugs to be used for this potentially life-threatening disease be tested rigorously so we have good evidence of their efficacy as well as their safety.
The trials represent a first-of-its-kind partnership among hepatology, infectious disease, critical care-pulmonology and cardiology departments at VCU. Sanyal leads the trials with a team of infectious disease doctors (Gonzalo M. Bearman, M.D., Michael P. Stevens, M.D., and Michelle E. Doll, M.D.), pulmonary critical care doctors (Marjolein de Wit, M.D., Lisa Brath, M.D., and Curtis N. Sessler, M.D.), researchers in inflammation (Antonio Abbate, M.D., Ph.D., and Benjamin Van Tassell, Pharm.D.) and VCU research and ethics staff.
“This has been really an exceptional team,” Sanyal said. “We were able to get the trial off the ground, meeting all of the complex regulatory requirements along the way, within a 72-hour time frame primarily because of the alignment of mission and vision and an extraordinary team effort from everybody involved.”
It is not one person’s trial, he added. The physicians, nursing staff, pharmacists and administrative staff came together to integrate the trial conduct with routine clinical care for eligible patients.
At VCU and VCU Health, the research represents just one response to a pandemic that has killed tens of thousands worldwide, including hundreds in the United States. With no approved medications to treat the virus currently, there is an urgent need for effective treatments.
“These clinical trials and the ongoing research of nationally prominent universities like VCU will quickly advance how health care teams treat COVID-19 around the world,” said VCU President Michael Rao, Ph.D. “We are proud of our research and health care teams, who are working hard to save lives and find solutions for patients with COVID-19.”
VCU Health is prepared for and is responding to the threat of the virus, and doctors are taking precautions to ensure the safety of all visitors, team members and patients, including those with suspected or confirmed cases of COVID-19 at the hospital. Those in the trials are isolated.
“Our team members at VCU and VCU Health have been actively searching for ways to combat COVID-19, a virus that has disrupted our lives and the lives of those we serve,” said Peter Buckley, M.D., dean of the VCU School of Medicine and interim senior vice president for VCU Health Sciences and CEO of VCU Health System. “Our teams are working seamlessly to make this investigational drug therapy available to those who are most affected by this virus.”
Sanyal, the principal investigator for the remdesivir trials, is a researcher at VCU Massey Cancer Center and the associate director of the KL2 program for training faculty in research at VCU’s C. Kenneth and Dianne Wright Center for Clinical and Translational Research, which oversees clinical trials at the university with the help of a $21.5 million National Institutes of Health grant.
The Wright Center, in collaboration with Sanyal’s team, developed a patient referral portal that allows doctors at other institutions to communicate with the trial team at VCU about possible participation for patients who meet the criteria — positive for COVID-19, hospitalized with a documented fever. If a patient from another health care provider qualifies, the patient could be eligible to transfer to VCU Medical Center to participate in the trial.
More information for researchers on these trials — for patients experiencing severe and moderate symptoms — is available at the National Institutes of Health.
Shawn Fenner, clinical research coordinator in VCU’s Division of Gastroenterology, Hepatology and Nutrition, contributed to this report.
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.